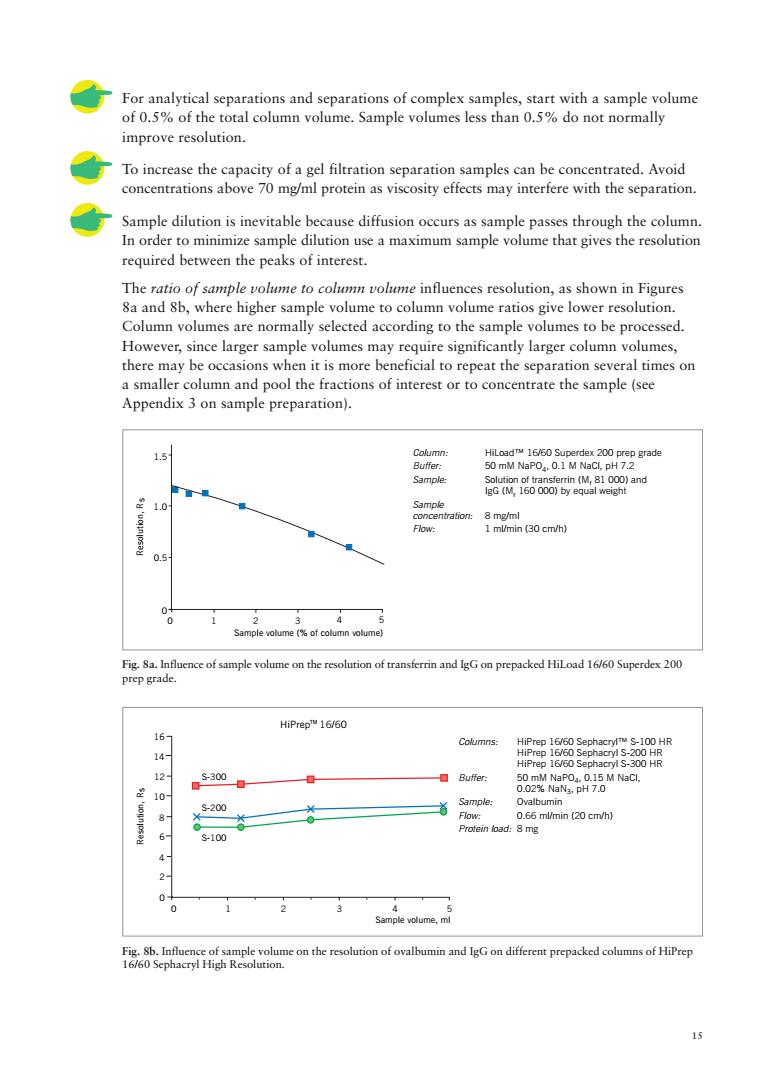
For analytical separations and separations of complex samples, of0.59% with a sample volume of th do not normally improve resolution To increase the capacity of a gel filtration separation samples can be concentrated.Avoid concentrations above 70 mg/ml protein as viscosity effects may interfere with the separation Sample dilution is inevitable because diffusion occurs as sample passes through the column In order to minimize sample dilution use a maximum sample volume that gives the resolution required between the peaks of interest. The ratio of sample volume to column volume influences resolution,as shown in Figures 8a and 8b,where higher sample volume to column volume ratios give lower resolution. Column volumes are normally selected according to the sample volumes to be processed. However,since larger sample volumes may require significantly larger column volumes, there may beneficial to repeat the sep aration several times on ons of interest a smaller t or t conc te the sample(see 0081o0and mlmin 00 cm/t 0.5 seonikinn HiPrep16/60 Columns: 12 s3006 ■Buer 50网 0 mlmin (2 cm) 6 0 IgG on different prepacked columns of HiPrep
15 For analytical separations and separations of complex samples, start with a sample volume of 0.5% of the total column volume. Sample volumes less than 0.5% do not normally improve resolution. To increase the capacity of a gel filtration separation samples can be concentrated. Avoid concentrations above 70 mg/ml protein as viscosity effects may interfere with the separation. Sample dilution is inevitable because diffusion occurs as sample passes through the column. In order to minimize sample dilution use a maximum sample volume that gives the resolution required between the peaks of interest. The ratio of sample volume to column volume influences resolution, as shown in Figures 8a and 8b, where higher sample volume to column volume ratios give lower resolution. Column volumes are normally selected according to the sample volumes to be processed. However, since larger sample volumes may require significantly larger column volumes, there may be occasions when it is more beneficial to repeat the separation several times on a smaller column and pool the fractions of interest or to concentrate the sample (see Appendix 3 on sample preparation). Fig. 8a. Influence of sample volume on the resolution of transferrin and IgG on prepacked HiLoad 16/60 Superdex 200 prep grade. Fig. 8b. Influence of sample volume on the resolution of ovalbumin and IgG on different prepacked columns of HiPrep 16/60 Sephacryl High Resolution. Sample volume (% of column volume) 0 123 4 5 0 0.5 1.5 1.0 Resolution, Rs Resolution, Rs Sample volume, ml 0 0 1 4 2 2 3 6 4 8 5 10 12 14 16 HiPrep 16/60 S-300 S-200 S-100 TM Column: HiLoad™ 16/60 Superdex 200 prep grade Buffer: 50 mM NaPO4, 0.1 M NaCl, pH 7.2 Sample: Solution of transferrin (Mr 81 000) and IgG (Mr 160 000) by equal weight Sample concentration: 8 mg/ml Flow: 1 ml/min (30 cm/h) Columns: HiPrep 16/60 Sephacryl™ S-100 HR HiPrep 16/60 Sephacryl S-200 HR HiPrep 16/60 Sephacryl S-300 HR Buffer: 50 mM NaPO4, 0.15 M NaCl, 0.02% NaN3, pH 7.0 Sample: Ovalbumin Flow: 0.66 ml/min (20 cm/h) Protein load: 8 mg

The beight of the packed bed affects both resolution and the time taken for elution.The resolution in gel filtration increases as the square root of bed height.Doubling the bed height gives an increase in resolution equivalent to2=1.4(40%).For high resolution ion long colum will give the best tesults and a bed height ber en30-60 should be satisfact Sufficient bed heightt resolution If a very long column is judged to be necessary,the effective bed height can be increased by using columns,containing the same media,coupled in series. Refer to Chapter 3 for detailed information on the theory of gel filtration. Media selection Chromatography media for gel filtration are made from porous matrices chosen for thei inertness and chemical and physical stability.The size of the pores within a particle and the particle size distribution are carefully controlled to produce a variety of media with different selectivities.Today's gel filtration media cover a molecular weight range from 100 to 80000 000,from peptides to very large proteins and protein complexes. The selectivity of a gel filtration medium depends solely on its pore size distribution and is described by a selectivity curve.Gel filtration media are supplied with information on their selectivity,as shown for Superdex in Figure 9.The curve has been obtained by plotting a partition coefficient K against the log of the molecular weight for a set of standard proteins (see Chapter 3 Gel filtration in theory for calculation of K). Superdex peptide 07s Superdex 75 Superdex 200 -Superdex 30 prep grade 0.5 -Superdex 75 prep grade -Superdex 200 prep grade 0.25 Fig.9.Selectivity curves for Superdex
16 The height of the packed bed affects both resolution and the time taken for elution. The resolution in gel filtration increases as the square root of bed height. Doubling the bed height gives an increase in resolution equivalent to È2 = 1.4 (40%). For high resolution fractionation long columns will give the best results and a bed height between 30–60 cm should be satisfactory. Sufficient bed height together with a low flow rate allows time for all 'intermediate' molecules to diffuse in and out of the matrix pores and give sufficient resolution. If a very long column is judged to be necessary, the effective bed height can be increased by using columns, containing the same media, coupled in series. Refer to Chapter 3 for detailed information on the theory of gel filtration. Media selection Chromatography media for gel filtration are made from porous matrices chosen for their inertness and chemical and physical stability. The size of the pores within a particle and the particle size distribution are carefully controlled to produce a variety of media with different selectivities. Today's gel filtration media cover a molecular weight range from 100 to 80 000 000, from peptides to very large proteins and protein complexes. The selectivity of a gel filtration medium depends solely on its pore size distribution and is described by a selectivity curve. Gel filtration media are supplied with information on their selectivity, as shown for Superdex in Figure 9. The curve has been obtained by plotting a partition coefficient Kav against the log of the molecular weight for a set of standard proteins (see Chapter 3 Gel filtration in theory for calculation of Kav). Fig. 9. Selectivity curves for Superdex. 0.25 0.50 0.75 1.00 10 100 1000 10000 100000 1000000 Kav Superdex peptide Superdex 30 prep grade Log Mr Superdex 200 Superdex 75 Superdex 200 prep grade Superdex 75 prep grade
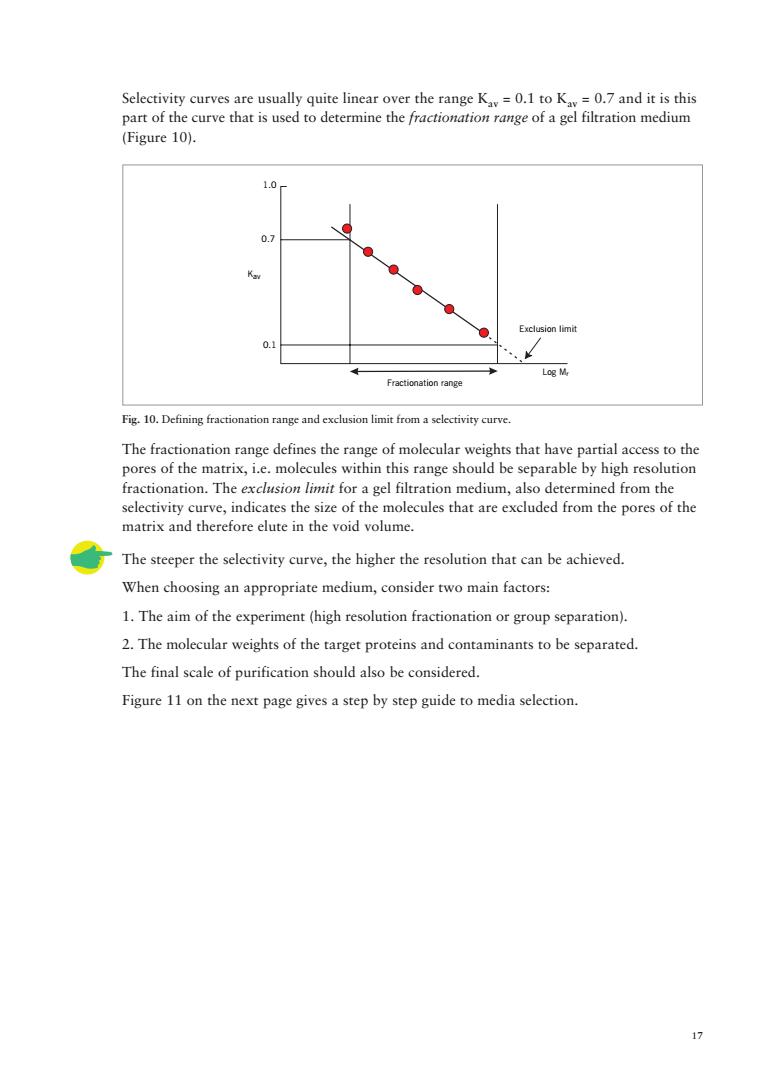
Selectivity curves part of t (Figure 10). 0 Eactsiomlm Log M Fractionation range Fig.10.Defining fractionation range and exdusion limit froma selectivity curve. The fractionation range defines the range of molecular weights that have partial access to the e matrix, thin this range should be onation.The from the pores of the The steeper the selectivity curve,the higher the resolution that can be achieved. When choosing an appropriate medium,consider two main factors: 1.The aim of the experiment (high resolution fractionation or group separation). 2.The molecular weights of the target proteins and contaminants to be separated. The final scale of purification should also be considered. Figure 11 on the next page gives a step by step guide to media selection
17 1.0 0.7 0.1 Fractionation range Exclusion limit Kav Log Mr Selectivity curves are usually quite linear over the range Kav = 0.1 to Kav = 0.7 and it is this part of the curve that is used to determine the fractionation range of a gel filtration medium (Figure 10). Fig. 10. Defining fractionation range and exclusion limit from a selectivity curve. The fractionation range defines the range of molecular weights that have partial access to the pores of the matrix, i.e. molecules within this range should be separable by high resolution fractionation. The exclusion limit for a gel filtration medium, also determined from the selectivity curve, indicates the size of the molecules that are excluded from the pores of the matrix and therefore elute in the void volume. The steeper the selectivity curve, the higher the resolution that can be achieved. When choosing an appropriate medium, consider two main factors: 1. The aim of the experiment (high resolution fractionation or group separation). 2. The molecular weights of the target proteins and contaminants to be separated. The final scale of purification should also be considered. Figure 11 on the next page gives a step by step guide to media selection
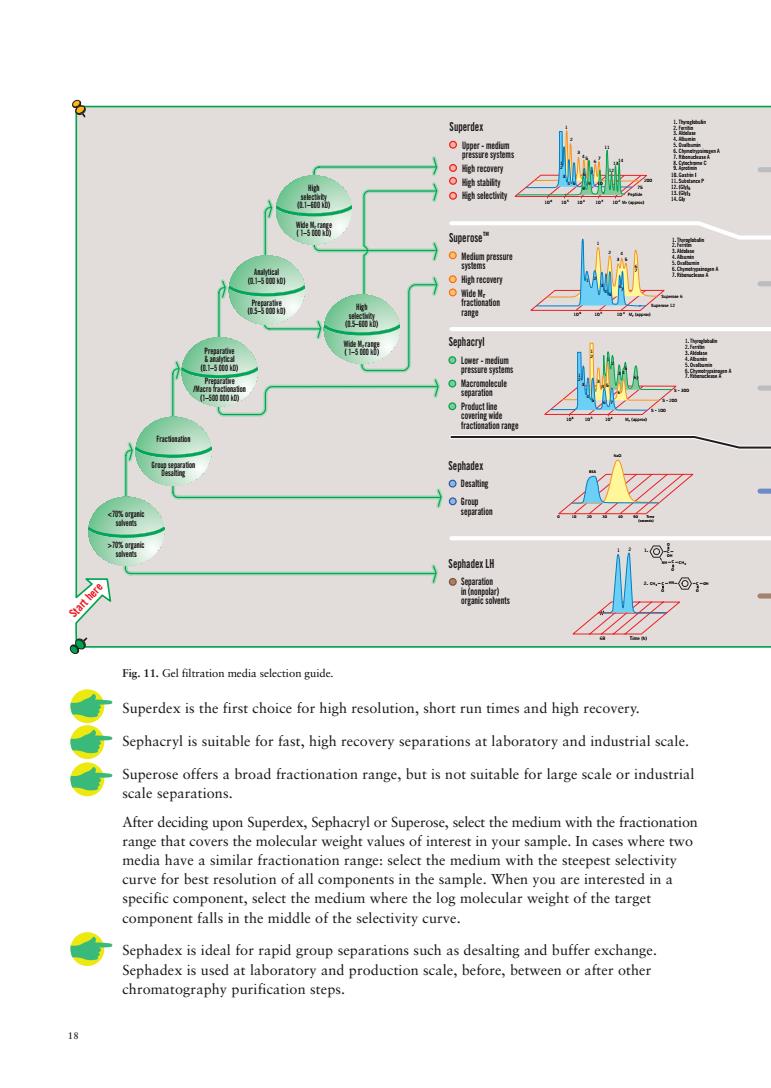
Fig.11.Gel fltration media selection guide. Superdex is the first choice for high resolution,short run times and high recovery. Sephacryl is suitable for fast,high recovery separations at laboratory and industrial scale. Superose offers a broad fractionation range,but is not suitable for large scale or industrial scale separations. After deciding upon Superdex,Sephacryl or Superose,select the medium with the fractionation range that co e ste curve for best of all c le whe rested in ent,select the Sephadex is ideal for rapid group separations such as desalting and buffer exchange Sephadex is used at laboratory and production scale,before,between or after other chromatography purification steps
18 Fig. 11. Gel filtration media selection guide. Superdex is the first choice for high resolution, short run times and high recovery. Sephacryl is suitable for fast, high recovery separations at laboratory and industrial scale. Superose offers a broad fractionation range, but is not suitable for large scale or industrial scale separations. After deciding upon Superdex, Sephacryl or Superose, select the medium with the fractionation range that covers the molecular weight values of interest in your sample. In cases where two media have a similar fractionation range: select the medium with the steepest selectivity curve for best resolution of all components in the sample. When you are interested in a specific component, select the medium where the log molecular weight of the target component falls in the middle of the selectivity curve. Sephadex is ideal for rapid group separations such as desalting and buffer exchange. Sephadex is used at laboratory and production scale, before, between or after other chromatography purification steps. Start here Superdex Upper - medium pressure systems High recovery High stability High selectivity Superose Medium pressure systems High recovery fractionation range Sephacryl Lower - medium pressure systems Macromolecule separation Product line covering wide fractionation range Sephadex Desalting Group separation Sephadex LH Separation in (nonpolar) organic solvents 250 mg 254mg 68 Time (h) 1 2 CH3 C C O O 2. C OH NH 3 O O 1. 10 20 30 40 50 Time (seconds) 0 BSA NaCl 1. Thyroglobulin 2. Ferritin 3. Aldolase 4. Albumin 5. Ovalbumin 6. Chymotrypsinogen A 7. Ribonuclease A 8. Cytochrome C 9. Aprotinin 106 105 104 1 2 3 4 5 1 3 4 5 6 7 7 6 2 3 4 5 6 1 2 10 6 10 5 10 4 10 3 10 2 1 2 3 4 5 6 7 8 9 10 11 12 1314 1 2 3 45 6 7 10. Gastrin I 11. Substance P 12. (Gly) 13. (Gly) 14. Gly 200 75 Peptide 1. Thyroglobulin 2. Ferritin 3. Aldolase 4. Albumin 5. Ovalbumin 6. Chymotrypsinogen A 7. Ribonuclease A 1. Thyroglobulin 2. Ferritin 3. Aldolase 4. Albumin 5. Ovalbumin 6. Chymotrypsinogen A 7. Ribonuclease A S - 300 S - 100 S - 200 10 6 10 5 10 4 1 2 3 4 5 6 7 4 5 2 3 1 6 7 Superose 6 Superose 12 <70% organic solvents Fractionation >70% organic solvents Group separation Desalting Preparative & analytical (0.1–5 000 kD) Preparative /Macro fractionation (1–500 000 kD) Preparative (0.5–5 000 kD) Analytical (0.1–5 000 kD) High selectivity (0.5–600 kD) Wide M range ( 1–5 000 kD) High selectivity (0.1–600 kD) Wide M range ( 1–5 000 kD) HN OH C CH r r Wide Mr M (approx) r M (approx) r M (approx) r 6 3 TM

range () 是 SephadexG-is for the majority of ns involving globular proteins. This medium is fo .Sephadex G-10 is well suited for the separation of biomolecules such as peptides (M,>700)from smaller molecules(M,>100). Sephadex G-50 is suitable for the separation of molecules M.>30 000 from molecules M,<1 500 such as labeled protein or DNA from free label. For group separations select gel filtration media so that high molecular weight molecules are eluted at the void volume,with minimum peak broadening or dilution and minimum time on the column.The lowest molecular weight substances should appear by the time one column volume of buffer has passed through the column
19 • Sephadex G-25 is recommended for the majority of group separations involving globular proteins. This medium is excellent for removing salt and other small contaminants away from molecules that are greater than Mr 5 000. • Sephadex G-10 is well suited for the separation of biomolecules such as peptides (Mr >700) from smaller molecules (Mr >100). • Sephadex G-50 is suitable for the separation of molecules Mr >30 000 from molecules Mr <1 500 such as labeled protein or DNA from free label. For group separations select gel filtration media so that high molecular weight molecules are eluted at the void volume, with minimum peak broadening or dilution and minimum time on the column. The lowest molecular weight substances should appear by the time one column volume of buffer has passed through the column. High resolution Fractionation range (globular proteins) 102 103 104 105 106 107 108 Superdex 30 prep grade Superdex 75 prep grade Superdex 200 prep grade Superdex Peptide Superdex 75 Superdex 200 Superose 6 prep grade Superose 12 prep grade Superose 6 Superose 12 Proteins Macro molecules Small proteins Proteins Large proteins Sephacryl S-100 HR Sephacryl S-200 HR Sephacryl S-300 HR Sephacryl S-400 HR Sephacryl S-500 HR Sephacryl S-1000 SF Sephadex G-10 Sephadex G-25 SF Sephadex G-25 F Sephadex G-25 M Sephadex G-50 F Exclusion limit Exclusion limit Exclusion limit Sephadex LH-20 Semi-preparative Analytical separation Preparative separation Preparative separation Semi-preparative Analytical separation Peptides Small proteins Polynucleotides Proteins DNA-fragment Peptides Small proteins Polynucleotides Proteins DNA-fragment Wide fractionation range Intermediate fractionation range Wide fractionation range Intermediate fractionation range Fractionation of macromolecules Purification of macromolecules Small particles Virus Small peptides Peptides/small proteins Proteins Low molecular steroids Terpenoids, lipids and peptides