
G山东工大写 Analytical chemistry As equilibrium is reached,we have R+00591gOX=. 0.059[OX]2 n [Red] n [Red]2 Ig K nDi 0.059 0.059 The conditional equilibrium constant K'can be used to calculate the completeness of a redox reaction.It can also be used to calculate the minimum difference in Ao'values needed for a quantitative reaction. 2022/11/19
Analytical chemistry As equilibrium is reached, we have The conditional equilibrium constant K' can be used to calculate the completeness of a redox reaction. It can also be used to calculate the minimum difference in Δφ0 'values needed for a quantitative reaction. 2022/11/19
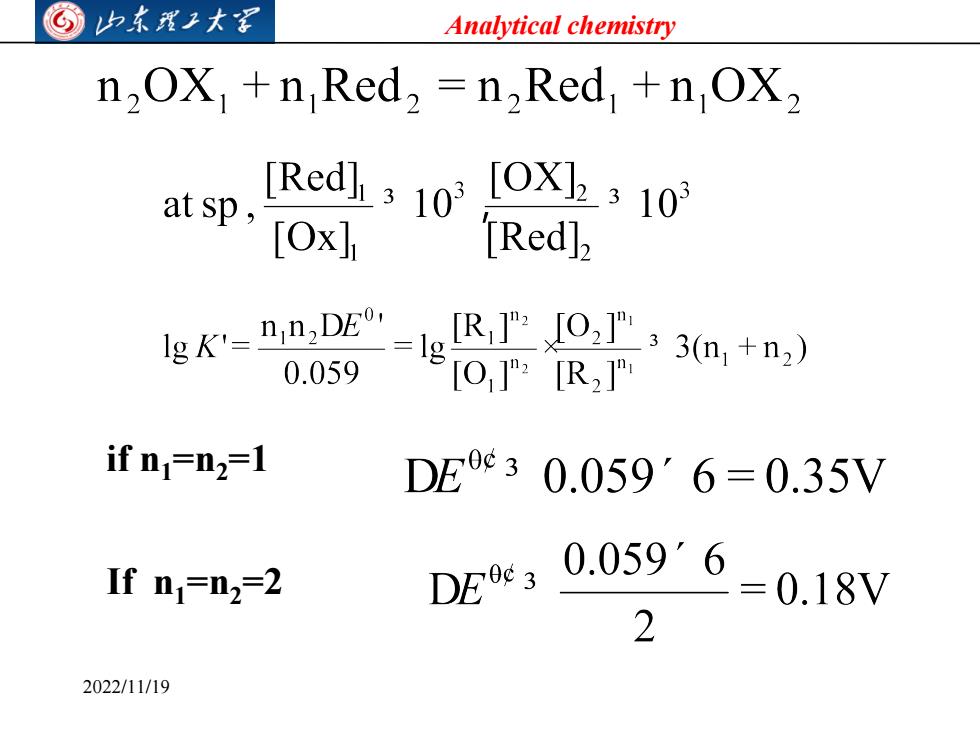
©⑤山东我工大军 Analytical chemistry n2OX +n Red2 =n2Red +n OX2 [Redl310I0X2310 at sp,TOxh Red]2 e太nS”e8R:a+ =1g 0.059 O]"?[R2 if n=n2=1 DEc30.059'6=0.35V If n=n2=2 DEt,0.0596-0.18y 2 2022/11/19
Analytical chemistry if n1=n2=1 If n1=n2=2 2022/11/19

归东理工大军 Analytical chemistry 6.2:Rate of redox reactions jece=1.44V>j50,1iso,=0.56V DEe >0.4V K-1029.8 But the rate of the reaction is very slow.There is no relationship between oo and rates because the two are different aspects of a reaction. The factors that affect the rate of a reaction are as follows 1.The concentration of the reactants Generally speaking,the reaction rate increases with increasing the concentration of the reactant. 2022/11/19
Analytical chemistry 6.2: Rate of redox reactions But the rate of the reaction is very slow. There is no relationship between φ0 and rates because the two are different aspects of a reaction. 1. The concentration of the reactants The factors that affect the rate of a reaction are as follows Generally speaking, the reaction rate increases with increasing the concentration of the reactant. 2022/11/19