
房束2黄大学 Guangdong University of Technology A51 Rendition of Charles Goodyear's 1839 invention of vulcanization (Rubber and Plastics News,August 1984)
8
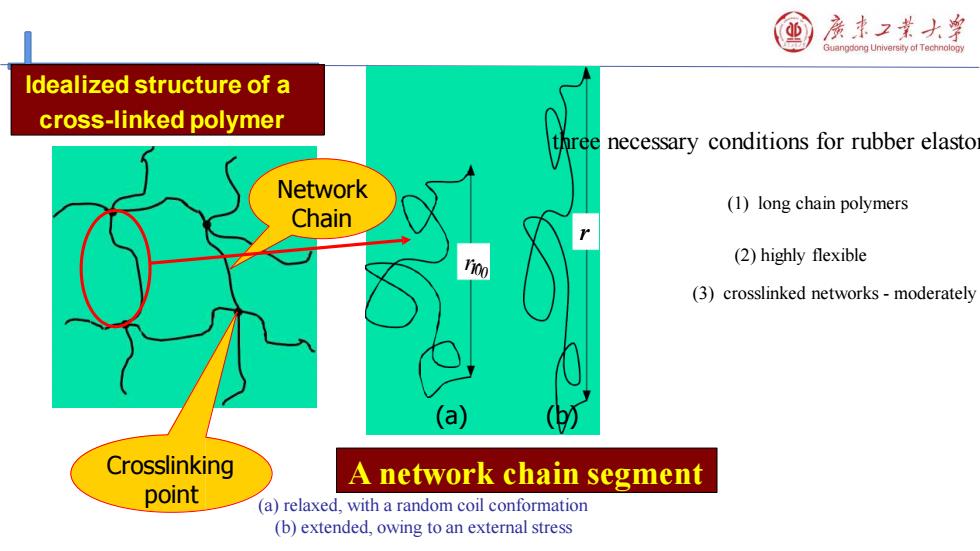
房事2黄大¥ Guangdong University of Technolopy Idealized structure of a cross-linked polymer three necessary conditions for rubber elasto Network (1)long chain polymers Chain (2)highly flexible (3)crosslinked networks-moderately (a) (0 Crosslinking A network chain segment point (a)relaxed,with a random coil conformation (b)extended,owing to an external stress
Idealized structure of a cross-linked polymer r0 r r0 A network chain segment Network Chain Crosslinking point (a) (b) (a) relaxed, with a random coil conformation (b) extended, owing to an external stress three necessary conditions for rubber elastomers: (1) long chain polymers (2) highly flexible (3) crosslinked networks - moderately crosslinked

房幸2黄大学 Guangdong University of Technology 橡胶态的本质?????? A simple rubber band may be stretched several hundred percent,yet on being released,it snaps back substantially to its original dimensions.By contrast,a steel wire can be stretched reversibly for only about a 1%extension.Above that level,it undergoes an irreversible deformation and then breaks.This long-range, reversible elasticity constitutes the most striking property of rubbery materials
橡胶态的本质?????? A simple rubber band may be stretched several hundred percent, yet on being released, it snaps back substantially to its original dimensions. By contrast, a steel wire can be stretched reversibly for only about a 1% extension. Above that level, it undergoes an irreversible deformation and then breaks. This long-range, reversible elasticity constitutes the most striking property of rubbery materials
房事2黄大学 Guanpdong University of Technolopy Characteristics of rubber (elastomer): When the size is stable and the deformation is small,the elastic response conforms to Hooke's law,which is similar to solid. .The coefficient of thermal expansion and isothermal compression are of the same order of magnitude as liquids --the intermolecular forces are similar to those of liquids; .The stress of deformation increases with temperature--similar to how the pressure of a gas increases with temperature
Characteristics of rubber (elastomer) : ● When the size is stable and the deformation is small, the elastic response conforms to Hooke's law, which is similar to solid. ● The coefficient of thermal expansion and isothermal compression are of the same order of magnitude as liquids -- the intermolecular forces are similar to those of liquids; ● The stress of deformation increases with temperature -- similar to how the pressure of a gas increases with temperature

房束2黄大学 Guangdong University of Technology thr ubber material porties loiqdorgas? solid liquid gas Certain appearance shape,The expansion coefficient of rubber is an The stress of deformation size stability,small order of magnitude larger than the general increases with the increase deformation in line with solid,the isothermal compression coefficient of temperature Hooke's law is similar to that of liquid,and the Possion Ratio is approximately equal to 0.5
solid liquid gas Certain appearance shape, size stability, small deformation in line with Hooke's law The expansion coefficient of rubber is an order of magnitude larger than the general solid, the isothermal compression coefficient is similar to that of liquid, and the Possion Ratio is approximately equal to 0.5 The stress of deformation increases with the increase of temperature