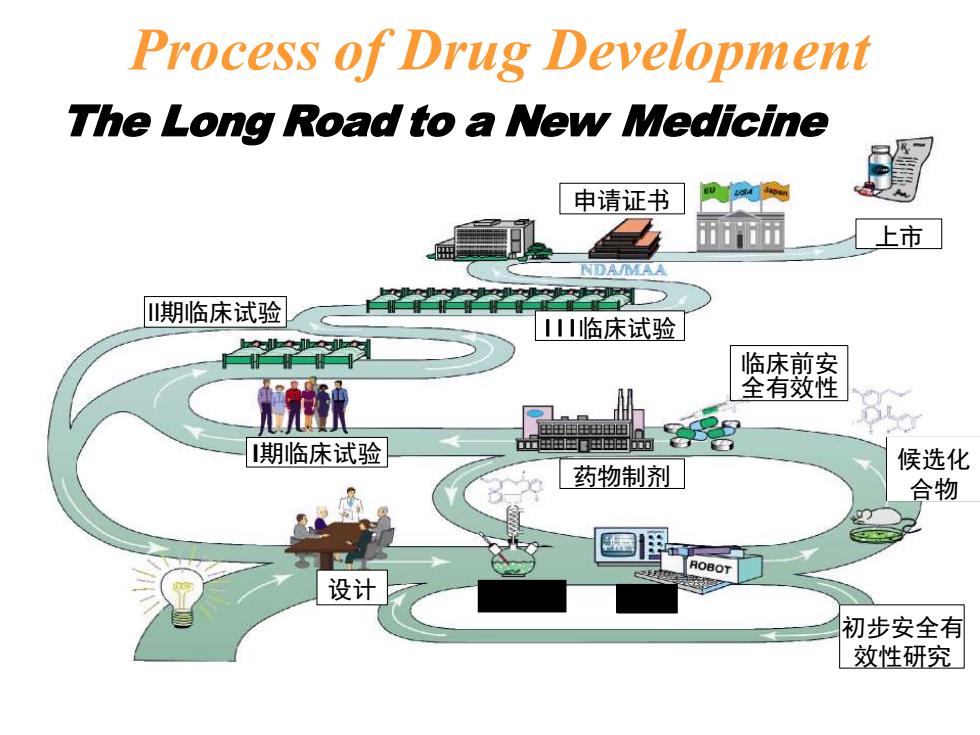
Process of Drug Development The Long Road to a New Medicine 申请证书 上市 NDA/MAA 山期临床试验 山临床试验 临床前安 全有效性 期临床试验 出出田田出由 药物制剂」 候选化 合物 ROBOT 设计 初步安全有 效性研究
The Long Road to a New Medicine I期临床试验 II期临床试验 III临床试验 临床前安 全有效性 药物制剂 候选化 合物 初步安全有 效性研究 设计 申请证书 上市 Process of Drug Development
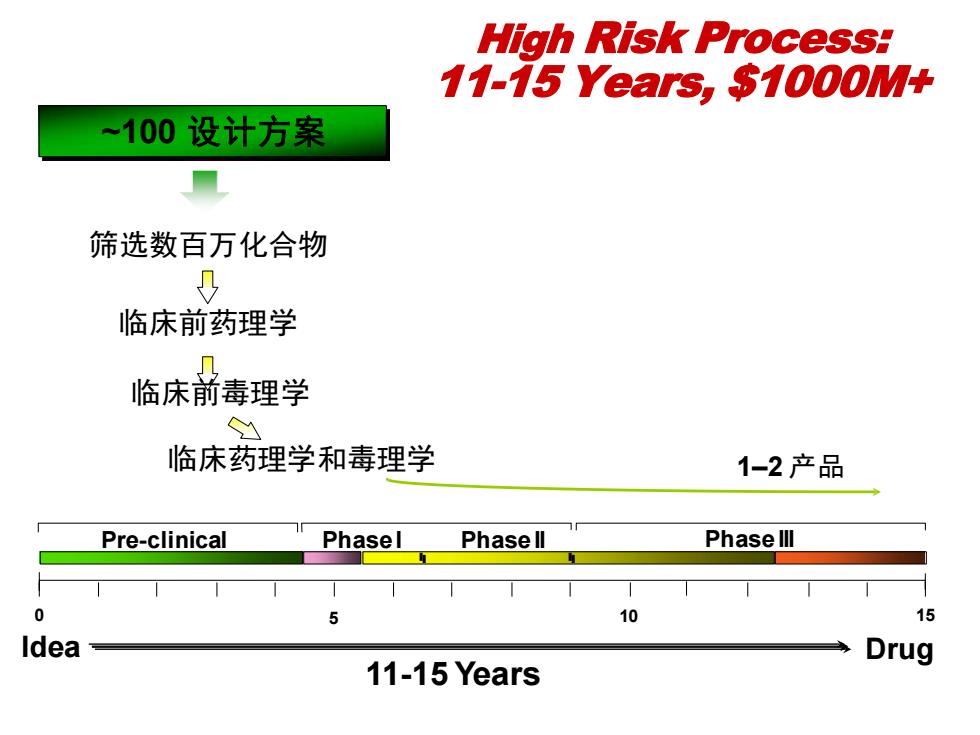
High Risk Process: 11-15 Years,$1000M+ ~100设计方案 ■ 筛选数百万化合物 临床前药理学 临床前毒理学 Y 临床药理学和毒理学 1-2产品 Pre-clinical Phasel Phase ll Phase lll 0 10 15 Idea Drug 11-15 Years
筛选数百万化合物 临床前药理学 临床前毒理学 11-15 Years 1–2 产品 Phase I Phase II Phase III 0 Idea 15 Drug 5 10 临床药理学和毒理学 ~100 ~100 设计方案 设计方案 Pre-clinical High Risk Process: 11-15 Years, $1000M+
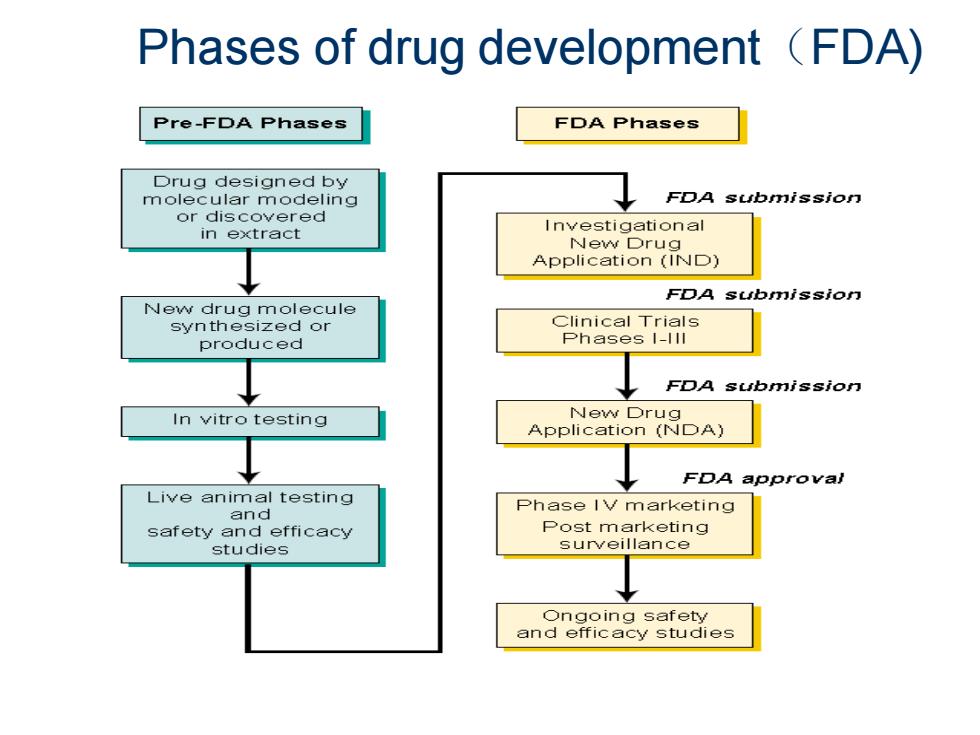
Phases of drug development (FDA) Pre-FDA Phases FDA Phases Drug designed by molecular modeling FDA submission or discovered in extract New Drug Application (IND) FDA submissio门 New drug molecule synthesized or Clinical Trials produced Phases I-lll FDA submissio门 In vitro testing New Drug Application(N□DA) FDA approval Live animal testing and Phase IV marketing safety and efficacy Post marketing studies surveillance ○ngoing safety and efficacy studies
Phases of drug development(FDA)
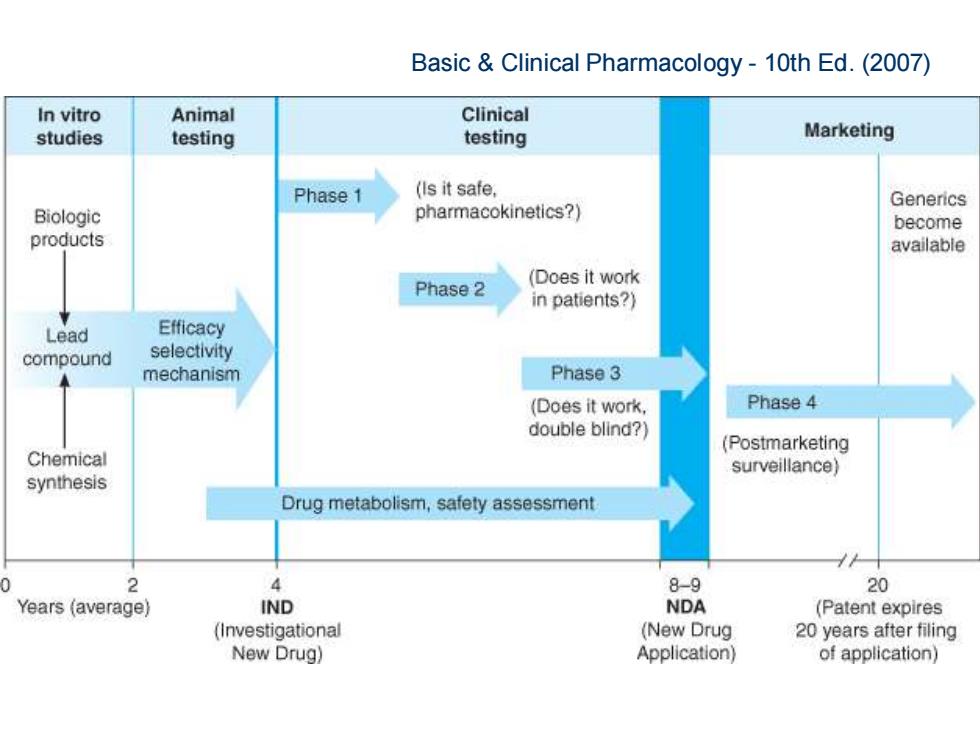
Basic Clinical Pharmacology-10th Ed.(2007) In vitro Animal Clinical studies testing testing Marketing Phase 1 (Is it safe. Generics Biologic pharmacokinetics?) become products available Phase 2 (Does it work in patients?) Lead Efficacy compound selectivity mechanism Phase 3 (Does it work, Phase 4 double blind?) (Postmarketing Chemical surveillance) synthesis Drug metabolism,safety assessment 2 4 8-9 20 Years (average) IND NDA (Patent expires (Investigational (New Drug 20 years after filing New Drug) Application) of application)
Basic & Clinical Pharmacology - 10th Ed. (2007)
格价药 此根合旧詩变! 脱柏换骨成新品 怎寨商家显种退。 又润隆价兴冲冲 2005年, 降价令 美国(FDA)共批准81 个新药上市,同期我国 SDA批准新药达1113个。 此中差距令人迷惑?! 药 推不动 图/张越新华社发
2005年, 美国(FDA)共批准81 个新药上市,同期我国 SDA批准新药达1113个。 此中差距令人迷惑?!